Conversion (high alumina cement)
-
29 October, 2024
-
2:18 pm
-
Conversion (high alumina cement)
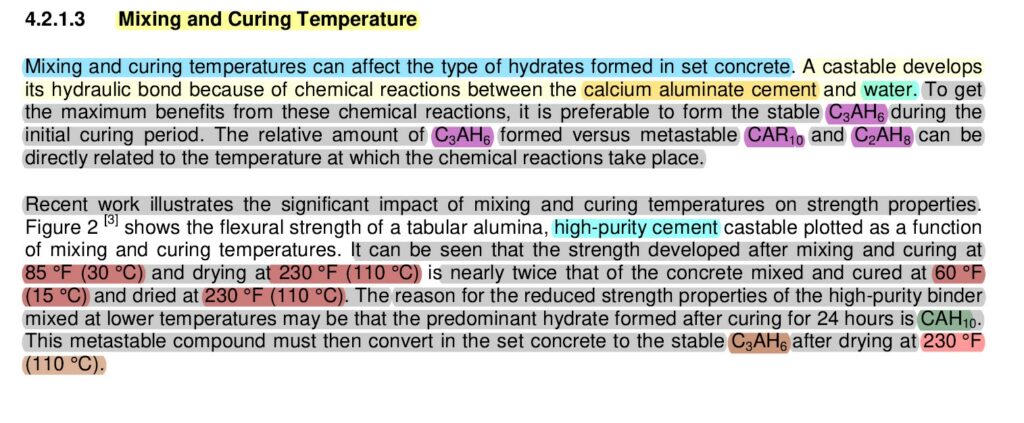
The mixing and curing temperature of calcium aluminate cement (CAC) refractory concrete has a significant impact on hydrate formation and, consequently, on the final material properties. The development of hydrate phases and their stability are strongly influenced by temperature, which affects the mechanical strength, durability, and chemical resistance of the refractory concrete.
Mixing Temperature:
- Cold Conditions (< 10°C):
- Slower Reaction: At lower temperatures, the hydration process is significantly slower. The formation of hydrates like CAH₁₀ (calcium aluminate hexahydrate) and C₂AH₈ (dicalcium aluminate octahydrate) is delayed.
- Longer Setting Times: The concrete may require more time to set and reach its initial strength.
- Risk of Cracking: Prolonged curing under these conditions might lead to improper hydration and cracking due to delayed development of strength.
- Optimal Conditions (20-30°C):
- Balanced Hydration: At these temperatures, CAC hydration is optimal, forming stable hydrates like C₃AH₆ (hydrous calcium aluminates) and AH₃ (gibbsite) without excessive rapid reactions.
- Good Strength Development: The material gains strength efficiently, and the microstructure develops with better durability.
- High Temperatures (> 30°C):
- Rapid Hydration: At high temperatures, the hydration process accelerates, leading to the formation of unstable hydrates such as C₂AH₈, which later decompose into C₃AH₆.
- Flash Setting Risk: If the mixing water heats up too quickly, flash setting can occur, leading to poor workability and lower long-term strength.
Curing Temperature:
- Low Curing Temperatures (0-10°C):
- Delayed Strength Development: At low curing temperatures, the formation of primary hydrates such as CAH₁₀ and C₂AH₈ is slowed down, and it may take longer to achieve desirable strength.
- Hydrate Stability: At temperatures below 10°C, hydrates like CAH₁₀ and C₂AH₈ remain stable, but they have lower long-term strength compared to other hydrates.
- Moderate Curing Temperatures (10-30°C):
- Stable Hydrates: At moderate temperatures, stable hydrates like C₃AH₆ form. This provides excellent mechanical strength and chemical stability, which is critical for refractory applications.
- Improved Properties: These conditions result in higher compressive strength, improved resistance to thermal cycling, and chemical attack.
- High Curing Temperatures (>30°C):
- Hydrate Transformation: At high curing temperatures, initially formed hydrates such as CAH₁₀ and C₂AH₈ convert into C₃AH₆, which has greater long-term stability and higher strength. However, this transformation can lead to dimensional instability (shrinkage and cracking) if not controlled.
- Thermal Decomposition: Extremely high temperatures can cause premature thermal decomposition of hydrates, reducing the material’s strength and resistance to high temperatures.
Hydrate Phases and Final Properties:
- Primary Hydrates at Low Temperatures: CAH₁₀ and C₂AH₈ form at temperatures below 20°C. These hydrates are metastable and gradually convert to C₃AH₆.
- Stable Hydrates at Moderate and High Temperatures: The formation of C₃AH₆ and AH₃ at higher temperatures leads to more durable refractory concrete with higher compressive strength and better resistance to high temperatures.
- Thermal Performance: Hydration and curing temperature affect the material’s performance at high service temperatures. Improper curing might result in the material being prone to degradation, spalling, or lower resistance to thermal cycling.
Summary of Key Effects:
- Low Temperatures: Slow hydration, extended setting time, and formation of weak hydrates.
- Optimal Temperatures (20-30°C): Balanced hydrate formation, good strength, and durability.
- High Temperatures (>30°C): Risk of rapid hydration, hydrate transformation, and possible dimensional instability.
For optimal performance, maintaining proper mixing and curing temperatures ensures the formation of stable hydrates that provide high mechanical strength, chemical resistance, and thermal durability essential for refractory applications.